Just like good practice guidelines for drug production, in order to meet GSP standards the business establishment must build hardware and software. In which hardware is a factory system, machinery and equipment. Software is a process system, document records.
3.1. Requirements for GSP-standard storage warehouse hardware

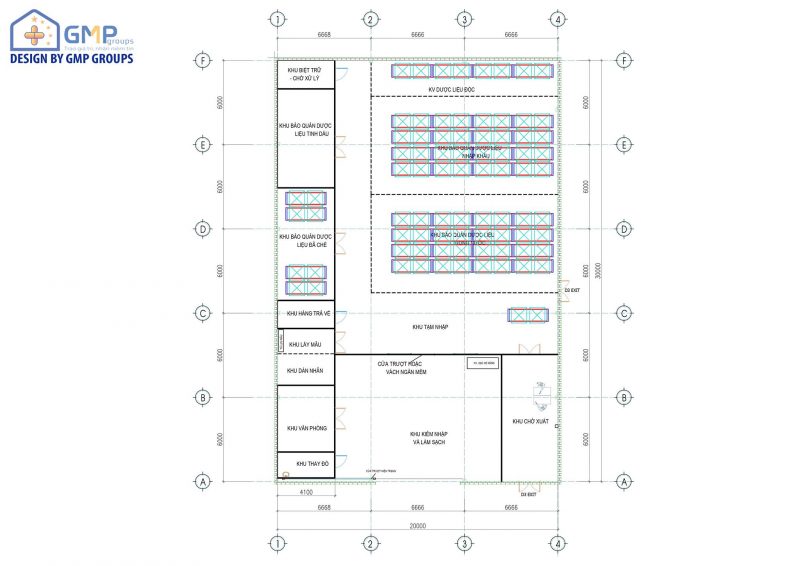
GSP Standard Storage Warehouse
Requirements for workshops and equipment are described in detail in Circular No. 36/2018/TT-BYT. In the scope of this introduction GMP Groups only emphasizes a number of important points:
a) The warehouse must have a certificate of fire prevention and fighting (FIREFIGHTING).
This is an important document in the inspection process, as well as a document that must submit a list of applications for GSP certification.
b) Functional areas must be clearly divided in accordance with GSP guidelines.
Storage warehouses must basically have the following functional areas:
– Warehouse office, costume replacement area
– Reception and cleaning area.
– Reserve area waiting for testing
– Approved material storage area
– Recovery-return storage area
– The storage area is removed.
– Sampling area
– Secondary labeling area (if necessary)
– Export area
c) The warehouse area must be sufficient for storage activities
Some preservation activities require a mandatory minimum area such as the preservation of medicinal herbs and traditional medicines.
d) The layout of the areas in the warehouse must be optimized in a convenient one-way direction during the storage process.
e) Ventilation and air conditioning systems must determine the appropriate cold and wind capacity to ensure product conditions as well as working environment for humans.
f) Areas for storage of specially controlled materials and drugs must comply with security control, avoid confusion and loss.
g) The warehouse must be tight-kept, avoiding the penetration of insects, termites, special rodents in the receiving and exporting area.
h) In order to meet the requirements of preventing termite insects and rodents in the warehouse, it is recommended to arrange mosquito-catching lights in open areas with external environments such as reception and export doors, ventilation doors must have insect nets installed, around the warehouse must plan and arrange traps for rodents …
i) Forklifts, trolleys, weighing equipment, humid thermometers… You have to invest enough. Weighing, humid heating must be periodically tested and calibrated. Besides the usual humid heat at each important storage warehouse, there is a self-recorded humidifier to monitor temperature and humidity online during storage.
k) The shelf system must be determined based on the load of goods to be preserved, rated as a shelf price to recognize and install the pricemeter must take into account the height of warehouses, human paths, goods. At each shelf, a list of goods showing the goods being preserved on the next rack must be listed.
l) The sampling area in the warehouse depends on the nature of the preserved material and finished product, which must be designed appropriately for sampling activities and maintain the quality of the product.

Form of certificate meeting GSP standards
3.2. Requirements for GSP-standard storage software
GSP-compliant storage document documentation process system is divided according to a stratified structure with 3 main levels
Level 1: the overall documents include: overall profile, quality handbook
Level 2: Instructions, operation processes, technical standards
Level 3: Records, drawings, external documents.
Documents and application processes at different levels must be built so that there is alignment and consistency in accordance with reality, so that when training and applying in practice must be strict, easy to understand, easy to comply with for people. From there, it will create a team of personnel with a strong quality management system.
In addition to investing in good infrastructure, facilities/enterprises must pay great attention to building a system of document records and training personnel to apply the operation of that document system.