GxP Standard Consultancy
The GxP standard is one of the services included in GMP Groups’ overall suite of cleanroom solutions.
The GxP standards were established by the U.S. Food and Drug Administration. The term surrounds many different regulations in many different areas.
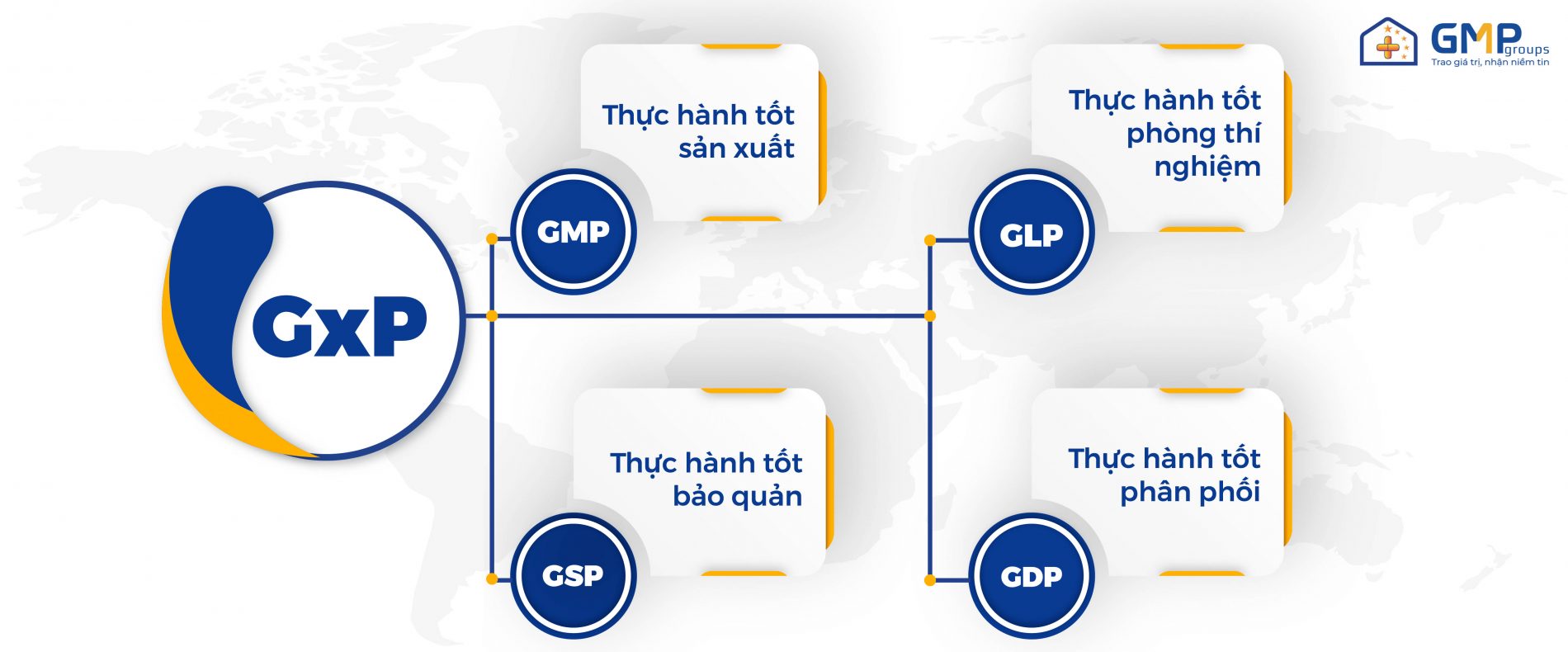
What is GxP? The letter G stands for “Good” and P stands for “Practice”. ‘X’ in the middle is a variable that can be replaced by any word that completes the acronym appropriately. Although the GxP regulations include many different sets of regulations, the most common are GMP, GLP, GSP and GDP.
The GxP standard includes:
– GMP Standard (Good Manufacturing Practice)
– GLP Standard (Good Laboratory Practice – Good Laboratory Practice)
– GSP Standard (Good Storage Practice)
– GDP standards (Good Distribution Practice – Good practice of drug distribution)