What is cleanroom appraisal and evaluation?
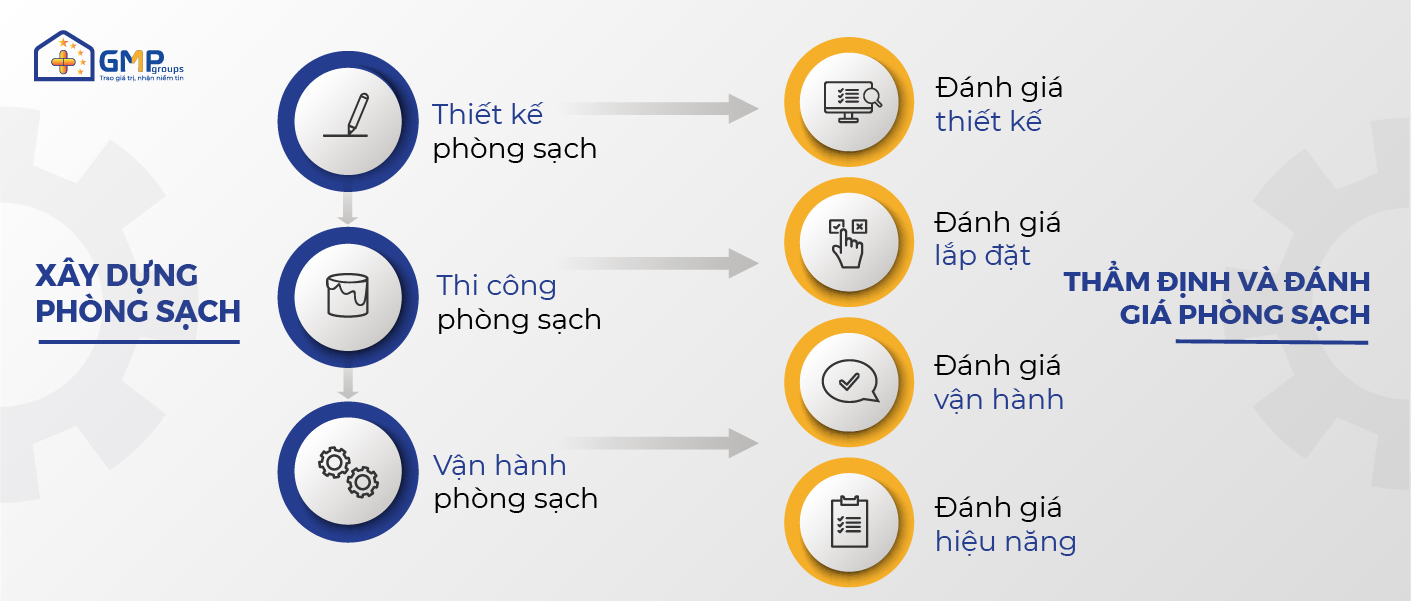
Clean room appraisal and evaluation is a service in GMP Groups’ overall suite of cleanroom solutions. The stages of cleanroom evaluation go hand in hand with the specific construction process as follows:
– Cleanroom Design Phase: Design Evaluation
The results obtained at the design stage are recorded in the “Design Evaluation Record”.
– Cleanroom construction phase: Installation assessment
The investor will conduct a practical assessment of the final construction and installation results, ensuring all changes in the design drawing have been updated in the finished drawing. At the same time, all such assessment and inspection activities are saved in the “Installation Assessment Dossier”.
– Cleanroom operation phase: Operational assessment, performance assessment.
+ Operational assessment is the evaluation and inspection to ensure all equipment and auxiliary of the cleanroom are in a normal state of operation. At the end of the operation assessment, the “Operation Assessment Dossier” is saved.
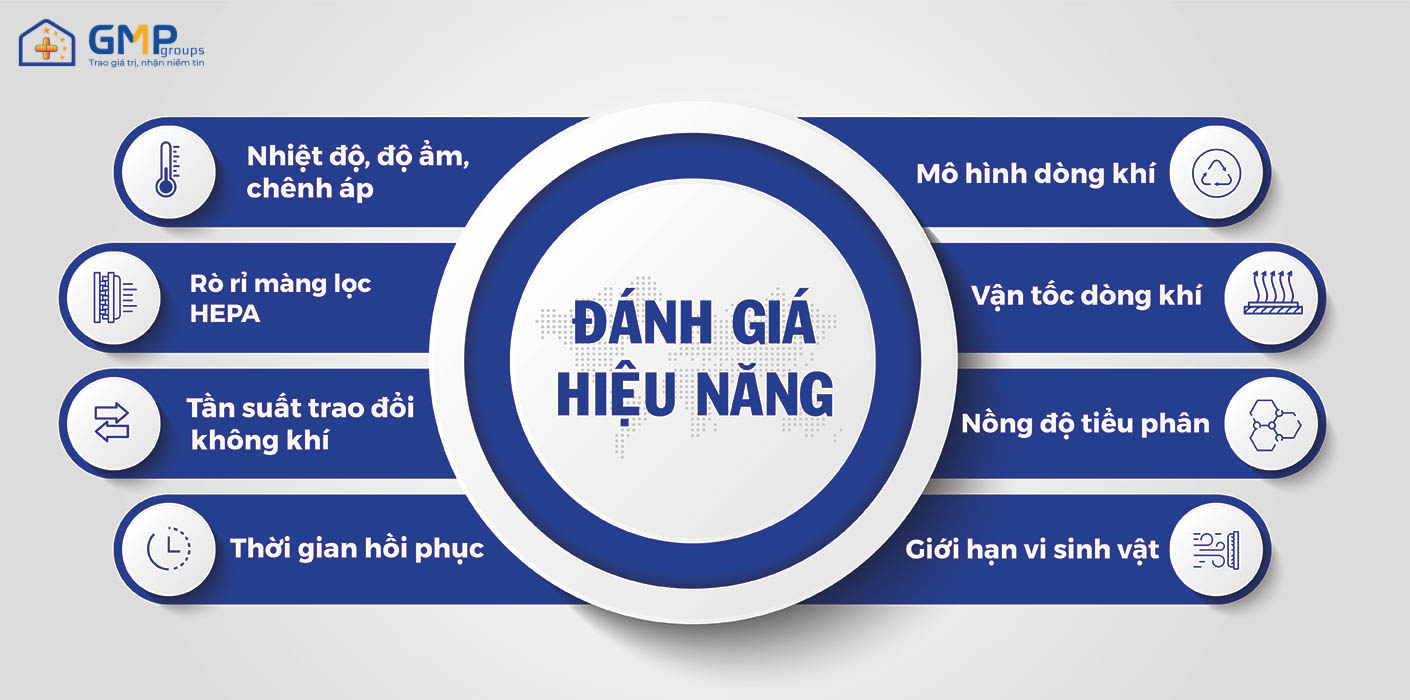
+ Performance assessment is the measurement of the environmental parameters of the cleanroom (urination, temperature, humidity, pressure difference, microbiological …) ensuring within the permissible limits. The results of the evaluation will be recorded in the “Performance Assessment Profile”