Organizations/businesses will take a lot of time and manpower if they study iso 13485 on their own. ISO 13485 sets out the conditions requirements to be met but does not specifically instruct the organization/business to do so.
Come to GMP GROUPS where there is a team of dedicated consultants, many years of experience, supporting the needs of customers: Supporting the procedure of classifying medical equipment (type A, B, C, D) and supporting the procedure of announcing eligibility for medical equipment production at the Department of Health. Support records and procedures for publication of applicable standards (for class A medical equipment) and product circulation announcements (for type B, C, D medical equipment).

GMP Groups advises ISO 13485 certification for Best Pacific Vietnam
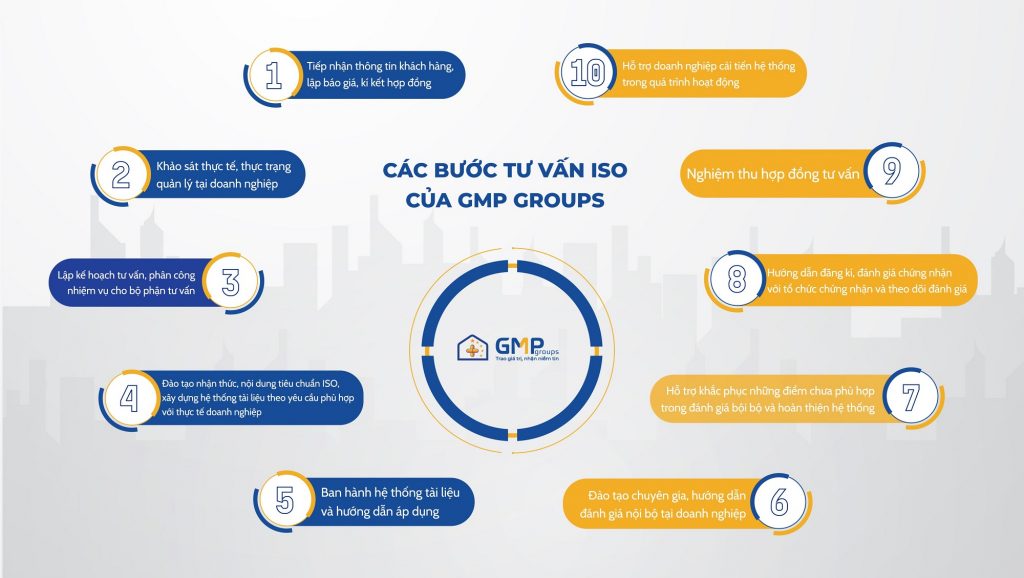
Choosing a suitable consulting unit to build you a quality management system of international standards to be able to implement management and supervision tasks well, bringing high efficiency is a necessary job to help your company and business be competitive in the market.

At GMP Groups, we offer ISO consulting services with the same standards:
ISO 9001:2015: Quality Management System
FSSC 22000: Food Safety System
ISO 22000: 2005 Food Safety Management System
ISO 17025:2005: Laboratory/Calibrate Quality Management
ISO 15189: 2012: Medical Laboratory Quality Management
HACCP Codex: Critical and critical checkpoint analysis
We commit to reasonable costs, fast time, maximum support and cutting fees incurred to deliver the most reasonable cost according to the size and status of the business. GMP Groups will be the orientation unit, helping businesses choose the right system as the evaluation process achieves ISO certification quickly and effectively to help businesses achieve the desired business efficiency.