Design and construction of cleanrooms in other fields
In addition to designing and constructing cleanrooms in key areas such as pharmaceuticals, cosmetics, food, veterinary and electronic, GMP Groups also provides an overall solution for cleanrooms in commonly applied fields such as:
Cleanrooms in the hospital sector
Cleanroom in the agricultural sector
Cleanroom in the field of research science
Hospital clean room
What is a hospital clean room?
Clean rooms are commonly used in surgical areas. Where wounds are open, parts of the human body are in direct contact with the external environment for long periods of time. Maintaining clean space in such cases is essential. Helps protect the body from environmental infectious agents; reduce the risk of infection or other risks that may occur due to environmental causes.
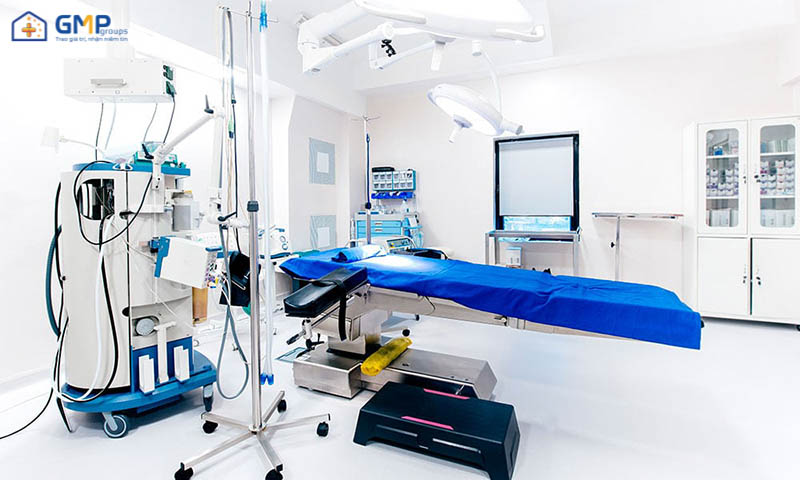
Hospital clean room
Requirements of the hospital cleanroom
The clean level of operating rooms in Vietnam is usually applied iso class 7. (according to the clean grade classification of ISO 14644-1:2015).
Design standards
The principle of designing cleanrooms for surgical areas in hospitals is similar to other cleanrooms. It is to minimize the accumulation of dirt through smooth surfaces; there are no cracks; the angles should be rounded, easy for cleaning and disinfection. The lamp should be flat with the ceiling; auxiliary systems for the operating room if repair and maintenance are needed should be designed so that it is accessible from the outside, avoiding implementation inside the surgical area.
The operating rooms should be designed in squares to easily arrange functions and machinery in the room. The corners of the room are designed to bevel 45o to facilitate the air circulation in the room.
In Vietnam, the operating room design standard requires a minimum area of 36m2, the height is not lower than 3.1m. In the United States, the hospital operating room is not smaller than 37m2, the width is not less than 6.1m, the height can be from 2.8m – 3.6m.
The design of clean rooms must adhere to the one-way principle to limit the phenomenon of contamination. The surgical cleanroom must have a 2-door system, a dedicated door for shift-band trolleys and an extra door.
Pressure requirements, microbiological limits
Depending on the nature of the surgery (normal or isolation) the pressure requirement in the operating room is positive or negative. Positive pressure applies to conventional surgery. Positive pressure applies to surgery that requires isolation.
Controlling air microorganism limits in surgical areas has a leading role. Because it directly affects the risk of infection during surgery. In addition to using an air treatment system with the types of filters needed to achieve the desired clean supply, air before entering the room should be passed through a UV sterilization system to ensure the microbiological limit is within the safest range.
Another very important factor in the design of a clean room for the surgical area is the high sound insulation requirements for the surgical team to focus on surgical expertise. Therefore, the materials used to build clean rooms must be carefully selected, of good quality limiting the penetration of pipes from the external environment.
Agricultural CleanRoom
Today, clean rooms are also widely used in the field of agriculture.
With the space strictly controlled the factors: temperature, humidity, urinary, microbiological … Cleanroom is the ideal environment for conducting experiments on plant-tissue culture, cultivating new varieties of plants thereby creating new varieties that are superior to traditional varieties.

Agricultural CleanRoom
Science Clean Room
To study a new drug, a new vaccine… Must be done in a cleanroom environment just like the one used to produce them. Therefore, in order for scientific research to be effective, research results and experiments with high accuracy in applying cleanrooms to the research process are extremely necessary.
The fields of scientific research often use cleanroom technology such as drug research, vaccines, experiments in the field of biology, chemistry …

Cleanroom in the field of science
GMP Groups – Unit of designing and constructing cleanrooms in all-inclusive fields
To ensure quality, clean rooms must be focused from the selection of locations and construction locations. Next is the design and construction phase. Finally, monitoring and due diligence. The market currently has many clean room construction units, but most units are only capable of providing one of the stages of cleanroom construction, making businesses take longer and more time and cost.
GMP Groups Services
GMP is proud to be the construction unit, designing clean rooms, capable of bringing the most comprehensive and synchronous service. Comprise:
– Consulting services, clean room design
– Cleanroom construction services
– Cleanroom assessment and appraisal services

GMP Groups – Prestigious cleanroom design and construction unit
Reasons to choose GMP Groups as a clean room construction and design unit
GMP Groups is trusted by investors as a cleanroom service provider by:
3D design system and VR-360 technology is a technological breakthrough of GMP Groups. This technology allows customers to experience the most vivid factory space and clean room right from the design stage.
– Experienced, enthusiastic, dedicated staff. GMP Groups’ employees are pharmacists and engineers who have been operating for 5-15 years in pharmaceutical companies.
GMP Groups understands the production technology of each dosage form. From there, we will provide our customers with the most effective and suitable design solution.
– We understand the requirements of cleanroom, clean supply, machinery and equipment- people in each stage of production. We can calculate for customers the requirements of area, air treatment system suitable, economical, most efficient.
With the desire to bring a comprehensive service ecosystem related to the clean sectors, GMP groups will be the perfect choice for any customer’s needs.
For more information on CONSULTING – DESIGN – CONSTRUCTION OF CLEANROOM, please contact:
GMP Groups Joint Stock Company
Head office: No 273 Hoa Ban, Ecoriver Eco-Investor, Hai Tan Ward, Hai Duong City, Hai Duong Province
Hotline: 0945.255.457 – Email: info@gmpgroups.com.vn – Web: gmpgroups.com.vn



