Thông tin dự án
| Project name | Construction project expanding phase 2: 5000m2 Dolexphar Factory |
|---|---|
| Investor | DOLEXPHAR NATIONAL PHARMACEUTICAL JOINT STOCK COMPANY |
| Location | Lot D and C-2 Dai An Expanded Industrial Park, Lai Way TT, Cam Giang District, Hai Duong Province |
| Scale | Workshop No. 1 of Syrup: 1,100 m2;
Workshop No. 2 produces effervescent tablets and probiotics: 1,800 m2 |
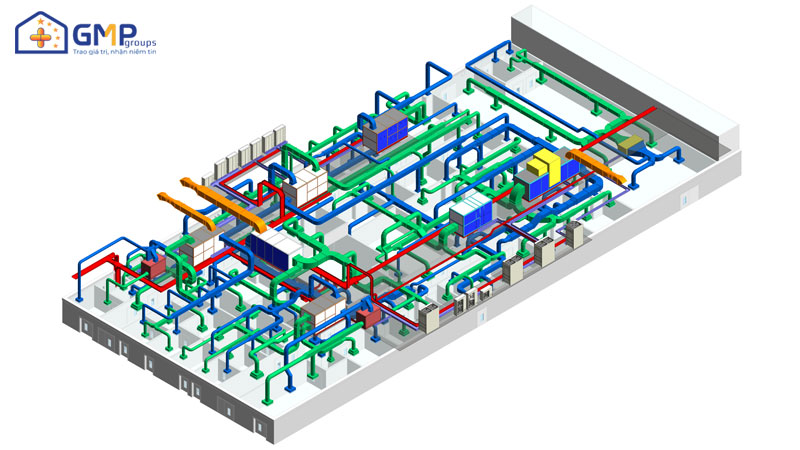
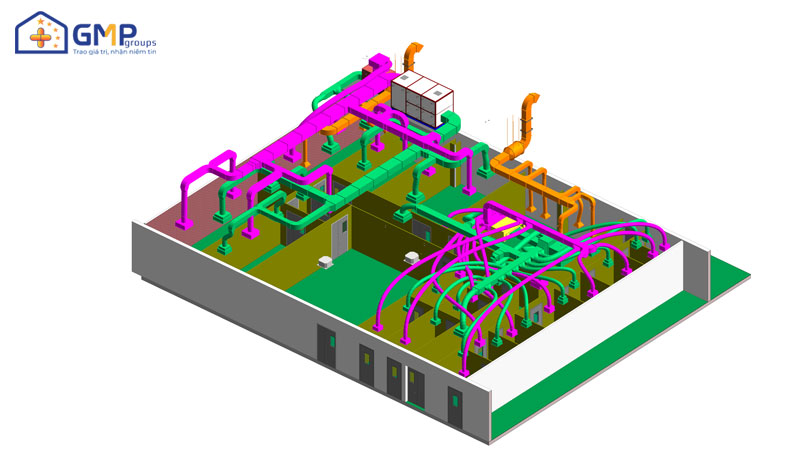
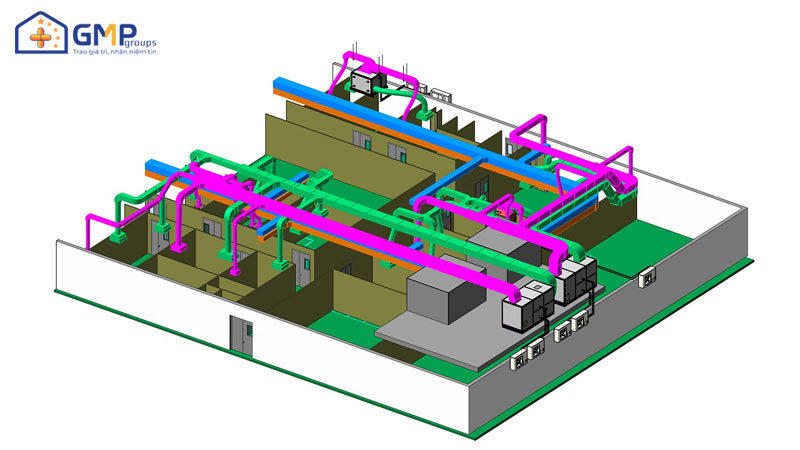
| Performing items | MEP system design &construction consultancy (Panel, HVAC, AHU, wind pipe system, air filtration, electrical system, Ro water, pneumatic system) |
| Rate of progress | Factory No. 1: July- September 2020 (Contract No. 008/2020/GMPGROUPS-DOLEXPHAR; May 28, 2020)
Factory No. 2: January – May 2021 (Contract No. 12/2020/GMPGROUPS-DOLEXPHAR; November 24, 2020) |
CONSTRUCTION AND EXPANSION PROJECT PHASE II OF 5000 M2 DOLEXPHAR FACTORY
The phase II construction and expansion project of Dolexphar factory is a project undertaken by GMP Groups as a consultant in the design and construction of MEP systems. This is considered a large project, contributing to proving the comprehensive capacity of GMP Groups in providing an overall set of cleanroom solutions for businesses.
Project context
Dolexphar International Pharmaceutical Joint Stock Company was established in 2016 with an investment of VND50 billion. Dolexphar operates in the field of formula consulting, food production of BVSK, cosmetics, medical equipment. In 2019, Dolexphar reached the top 10 GMP-HS-standard factories in Vietnam. With the desire to scale up, with the goal of capturing the market share of health-protecting food of the whole North, Dolexphar has decided to expand the scale and diversify the production line. To achieve that goal, Dolexphar looks forward to finding an MEP contractor capable of providing a comprehensive, efficient, comprehensive MEP solution. Among many MEP solution providers in the market, GMP Groups is trusted by Dolexphar’s Board of Directors as a contractor to design and comprehensively construct the MEP system.
Challenges, difficulties
During the 14 months of construction of the project, the construction team of GMP Groups faced many difficulties such as:
Harsh construction environment, hot summer weather, up to 50 degrees Celsius;
The project requires high accuracy, mandatory GMP-HS standards and other standards in the construction of clean rooms;
Construction workers on the EPS floor have deteriorated, the relevant old system is carried out by the inexperienced;
The construction must ensure safety, hygiene and care to avoid affecting the nearby production activities;
Covid-19 conditions require GMP Groups workers to join hands to fight the epidemic with localities and increase shifts to speed up the work.
Project implementation process
The process of implementing the construction and expansion project phase II of Dolexphar factory goes through the following main stages:
Consulting phase
After receiving project information, GMP Groups conducted a field survey and held many meetings with the Investor, thereby offering advice on options:
– Consulting with suitable technology lines: syrup, medicines, effervescent drugs
– Selection of suitable supplies and equipment for building clean rooms meeting GMP standards with the lowest cost
– Selection of production equipment, testing equipment, optimal warehouse equipment
Design phase
From the results of the survey of the current status of the project, contact with investors to accurately calculate the needs and costs of use, GMP Groups has come up with a safe design plan, suitable for the construction of ANG-HS factory and the optimal technical plan on both technical and economic perspectives.
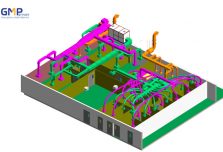
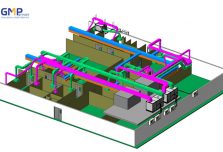
Structural: The project belongs to phase II, consisting of 3 workshops: Frozen syrup factory, Microbiological Medicine Workshop, Effervescent Nui nucucule workshop and auxiliary areas.
In terms of scale: The project is invested to build on an area of 5,000m2 (0.5 ha) of which the scale of 2 main workshops is: Syrup drug factory: 1100m2; Effervescent tablets and probiotics: 1800m2.
In particular, GMP Groups uses technological breakthroughs in design such as VR360 technology to help investors visit the whole project visually even without construction, timelapse technology helps investors monitor the progress of the project in the most effective way.
Construction phase
After agreeing on the design plans, in November 2020, the construction project of The Dolexphar Factory was started construction.
Thanks to a team of professional engineers and skilled workers, GMP Groups has completed 5 system items: Epoxy Floor, Pannel, Electricity, HVAC, Auxiliary. Each item ensures the criteria of quality, safe operation, savings and efficiency.
Stage of training, acceptance and handover of works
After completing the project, GMP Groups continuously tested the whole system, especially the AHU system to weigh pressure and check the tightness of the room and door, check the temperature and humidity, check the system durability.
GMP also organizes operational training for the electromechanical team of dolexphar investor. At the end of the training period, GMP Groups conducted the acceptance and handover of the works with detailed reports.
Stage of Maintenance and Servicing
After handing over the works, GMP Groups continues to support Dolexphar in warranty and maintenance whenever there are technical problems.
With the outstanding advantages that have been proven through reality, GMP Groups’ design and construction services have been affirmed, GMP Groups brand is increasingly chosen by investors.
Regardless of the different field and scale, the solutions for designing and constructing clean rooms are carefully implemented by GMP Groups engineers and architects, the most suitable, meeting the right and best according to the requirements of each investor.
See more GMP Groups works:
– Health Food Factory Project – FDA
For more information about TU Q&A – OWNERSHIP – CONSTRUCTION OF CLEANROOMS, please contact:
GMP Groups Joint Stock Company
Head office: No 273 Hoa Ban, Ecoriver Eco-Investor, Hai Tan Ward, Hai Duong City, Hai Duong Province
Hotline: 0945.255.457 – Email: info@gmpgroups.com.vn – Web: gmpgroups.com.vn