GxP & ISO Standard Consulting Services
One of the biggest concerns of the pharmaceutical, cosmetic, food and medical devices industries. It’s the quality of your products. The GxP standard is a set of regulations and guidelines to solve this problem systematically and effectively. Join GMP Groups to learn more about the GxP standard in the article below.
- 1 What is the GxP & ISO standard?
- 1.1 GMP Standard (Good Manufacturing Practices- Good Manufacturing Practices)
- 1.2 GLP Standard (Good Laboratory Practice – Good laboratory practice)
- 1.3 GSP Standard (Good Storage Practices – Good practice of preserving medicines)
- 1.4 ISO Standards (International Organization for Standardization – International Standardization Organization)
- 2 GxP &ISO Quality Management System Consulting Service at GMP Groups
What is the GxP & ISO standard?
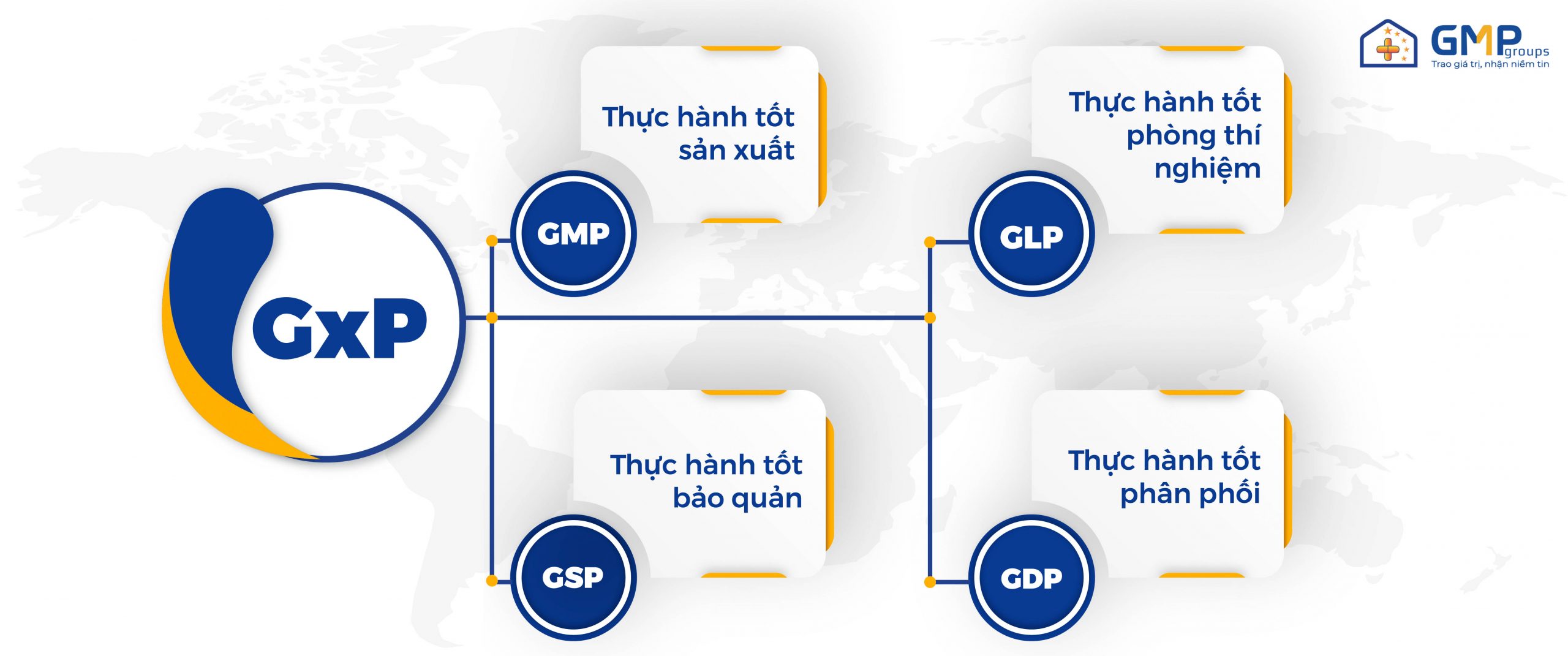
The GxP standards were established by the U.S. Food and Drug Administration. The term surrounds many different regulations in many different areas. What is GxP? The letter G stands for “Good” and P stands for “Practice”. ‘X’ in the middle is a variable that can be replaced by any word that completes the acronym appropriately.
These regulations and guidelines focus primarily on:
– Product traceability
– Accountability
– Data integrity
Although the GxP regulations include many different sets of regulations, the most common are GMP, GLP, GSP and GDP.
GxP and ISO standard consultancy
GMP Standard (Good Manufacturing Practices- Good Manufacturing Practices)
GMP is part of a quality management system that ensures factory conditions (infrastructure), human conditions and controls production processes to meet the standards of hygiene and safety provided to consumers, eliminating the risk of cross-contamination and confusion.
GLP Standard (Good Laboratory Practice – Good laboratory practice)
GLP is a set of principles and standards on quality management system related to the process of organization and conditions for conducting non-clinical research in pharmaceutical activities for human health and environmental safety that are planned, implemented, monitored, recorded, storage and reporting.
GSP Standard (Good Storage Practices – Good practice of preserving medicines)
GSP is a set of principles and standards relating to appropriate measures for the preservation and transportation of raw materials and products at all stages of production, storage, storage, transportation and distribution of drugs in order to ensure and maintain the best safety and quality of drugs, medicinal materials through adequate control throughout the storage process.
GDP standards (Good Distribution Practices – Good practice of drug distribution)
This standard sets out strict requirements to ensure the comprehensive quality of drugs during the transportation and distribution of drugs to ensure the supply of drugs to consumers in a timely, complete and quality manner.
ISO Standards (International Organization for Standardization – International Standardization Organization)
Not only does it stop at meeting the standards of domestic law, but businesses also try to meet international standards including ISO standards.
ISO is an international set of standards set by ISO to make everything done in accordance with the standards including providing international standard specifications to ensure products, services and systems, ensuring quality, safety and efficiency.
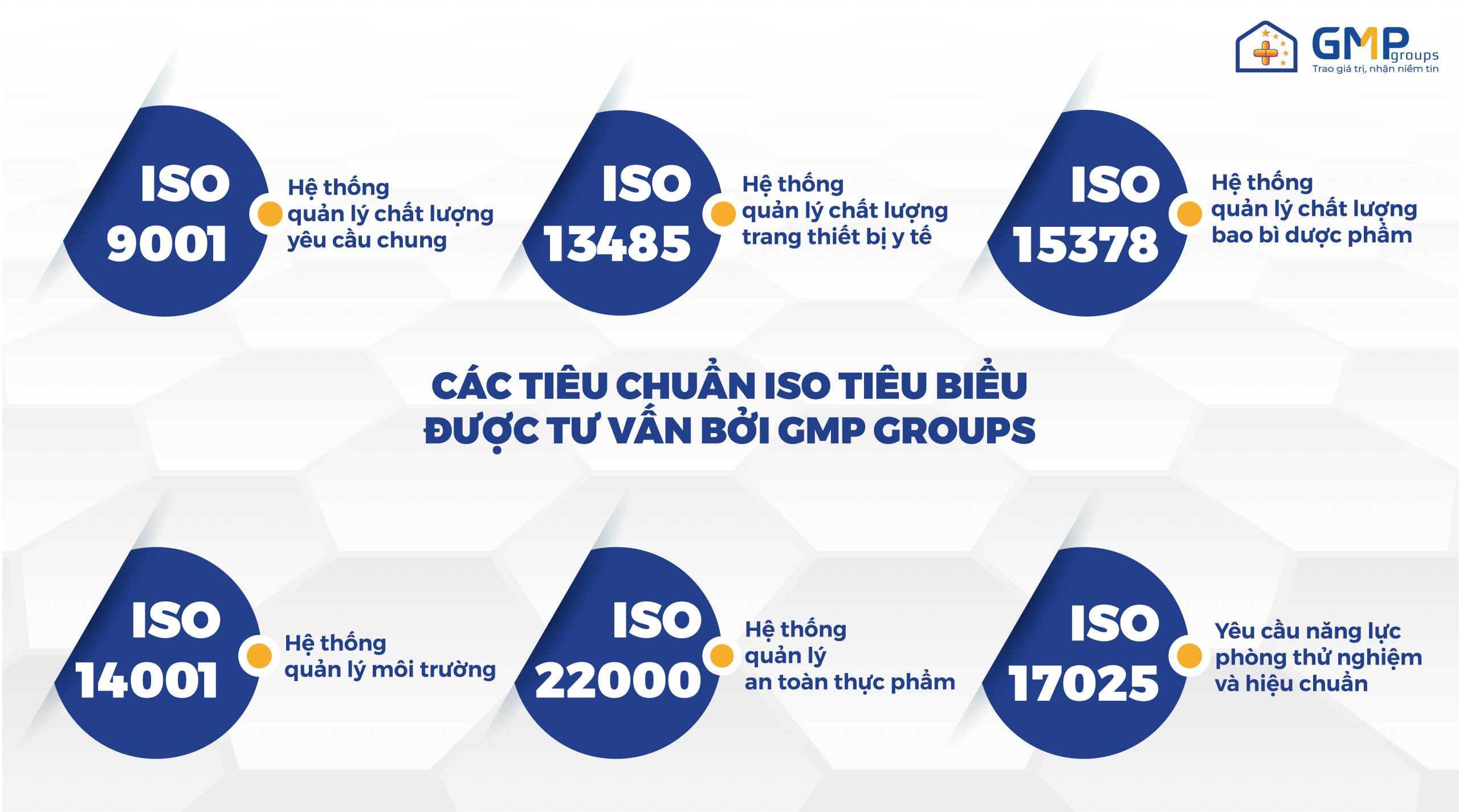
Some of iso’s common standards such as:
Typical ISO standards are advised by GMP Groups
GxP &ISO Quality Management System Consulting Service at GMP Groups
Building a quality management system is not easy. This requires investment in people, equipment and related procedures. Therefore, most businesses need the support and advice of a specialized unit in the field of quality management consultancy.
As a company operating for many years in the field of quality management consultancy, GMP Groups has a team of experts who are experienced pharmacists and engineers leading in the quality system of GMP, GSP, GLP & ISO system deployment.
GMP GROUPS ensures comprehensive consultation of GMP, GSP, GLP &ISO systems until certification is received including:
– GxP Standard Consultancy
+ Consulting on completing the premises of production, testing and warehouse technology
+ Consulting to complete the overall premises, investment items in accordance with the regulations to meet the requirements of customers
+ Catalog equipment for production (GMP), testing (GLP) and warehouse (GSP)
+ Supporting the selection of equipment, tools and consumables for the factory
– Basic training of GMP/GLP/GSP/GDP/ISO standards
+ Develop a training plan
+ General training in GMP/GLP/GSP/GDP/ISO
+ Training knowledge on system appraisal: Assessment of HVAC system, production environment, domestic water system, RO water, distilled water, pneumatic, storage conditions, equipment appraisal, production process appraisal, hygiene process appraisal
– Instructions for drafting dossiers and documents
+ Cataloging of SOP documents and dossiers
+ Instructions for drafting SOP systems related to GMP/GLP/GSP/GDP/ISO
+ Develop the original appraisal plan
+ Guidance on building IQ, OQ, PQ for production equipment, HVAC system, RO water treatment system, distilled water.
+ Build a water quality handbook
+ Guidance on the development of a scheme for appraisal of the production environment
+ Guidance on building GLP/GSP/ISO-related SOPs

GMP Groups’ quality management system consulting team
– Completing the application for evaluation and certification of GxP standards
+ Complete IQ, OQ, DQ assessments for equipment, HVAC system, water, pneumatic
+ Guidance on production and testing of 03 appraisal lots, guidance on completing appraisal of production process
+ Instructions for completing the application for evaluation
– Conduct self-inspection and overcome the existence after self-inspection
– Overcoming inappropriate points after the evaluation team assesses GMP/GLP/GSP/GDP
– Together with the investor maintains the system of documents according to GMP/GLP/GSP/GDP for the next assessment.
Refer to some typical certifications we have advised at: Quality management system standard certification
For any questions related to GxP & ISO standard consultancy in general and details of quality management systems, please contact GMP Groups for direct support from experts.
GMP Groups Joint Stock Company
Head office: Lot LK20.8, Ecoriver Eco-Investor, Hai Tan Ward, Hai Duong City, Hai Duong Province
Hotline: 0945.255.457
Website: gmpgroups.com.vn
Email: info@gmpgroups.com.vn



