Cosmetic production conditions
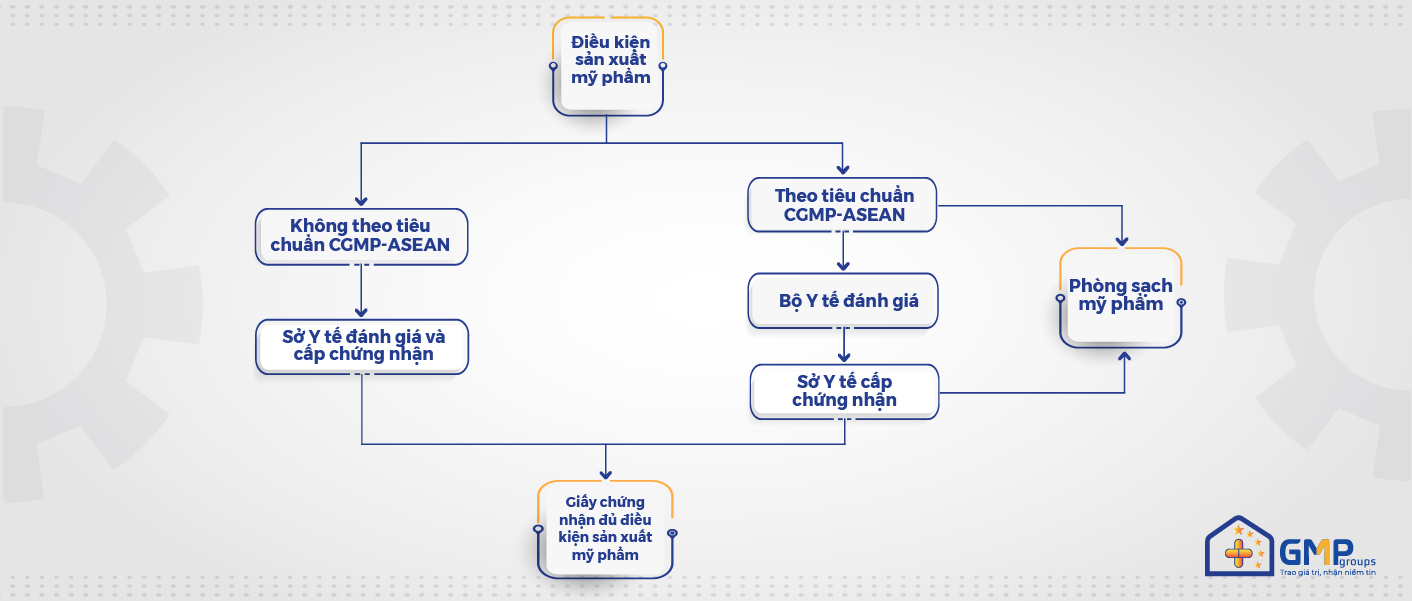
Cosmetics in Vietnam are becoming more and more tightly controlled over time. Currently, establishments must meet certain production conditions in compliance with Decree 93/2016/ND-CP and Decree 155/2018/ND-CP, which are evaluated and certified by the Department of Health.
Especially to ensure the safety and quality of products as well as products allowed to trade not only domestic markets but also in asean associations, compliance with the principles and standards of ‘Good Practice guidelines for cosmetic production’ (cGMP-ASEAN) is a mandatory requirement.
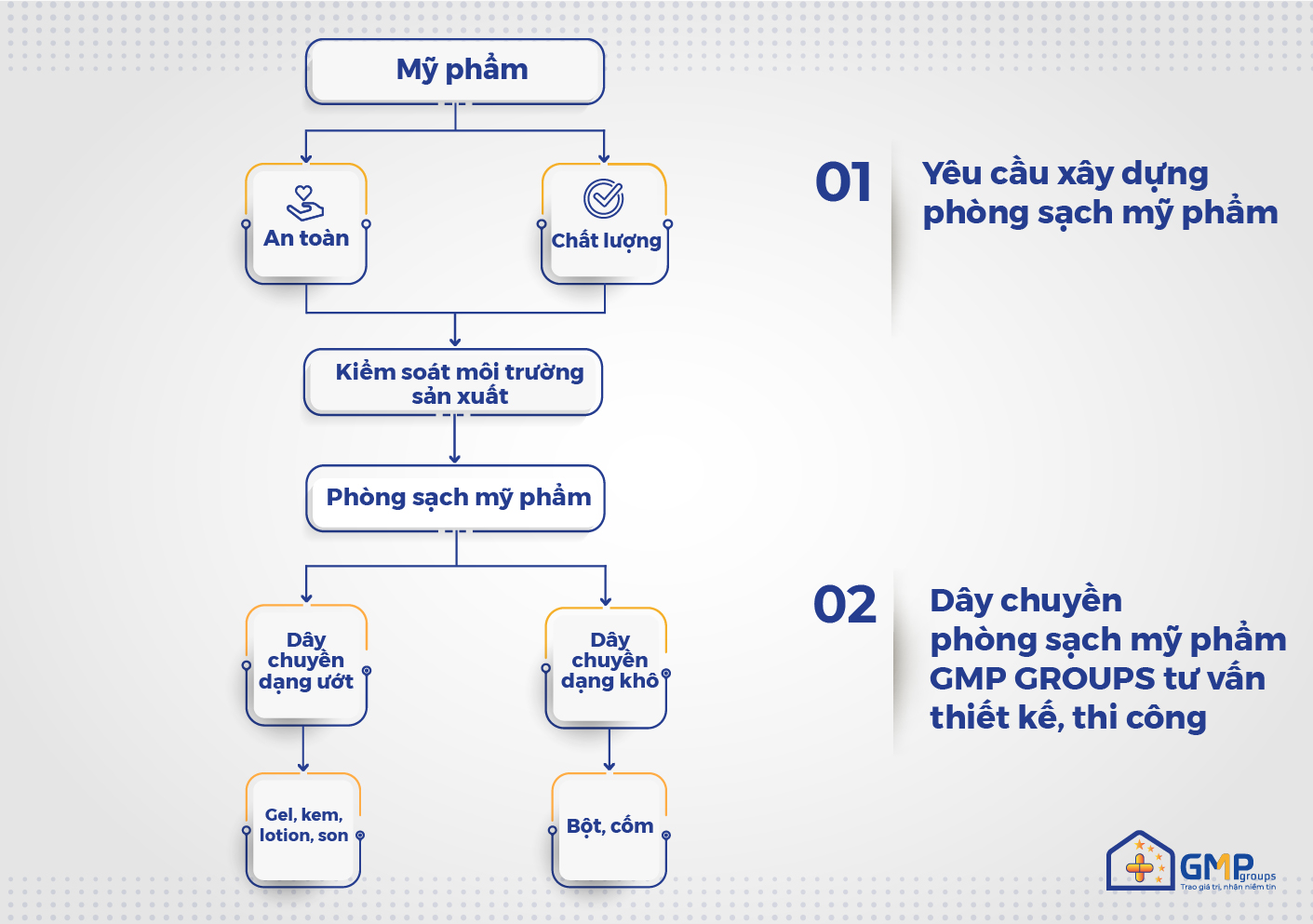
Cleanliness in cosmetic production is not specifically mentioned in the guidance systems or principles and standards of “good cosmetic manufacturing practice guidelines” (cGMP-ASEAN). But the majority of D-levels (equivalent to ISO class 8 as classified in ISO 14644-1:2015) are applied in pharmaceutical manufacturing to cosmetic manufacturing applications. In addition, depending on the quality control requirements of the manufacturer, higher clean levels can be applied.