Construction requirements and cleanroom lines GMP Groups consulting design and construction
2.1. Requirements for the construction of a clean room for veterinary medicine
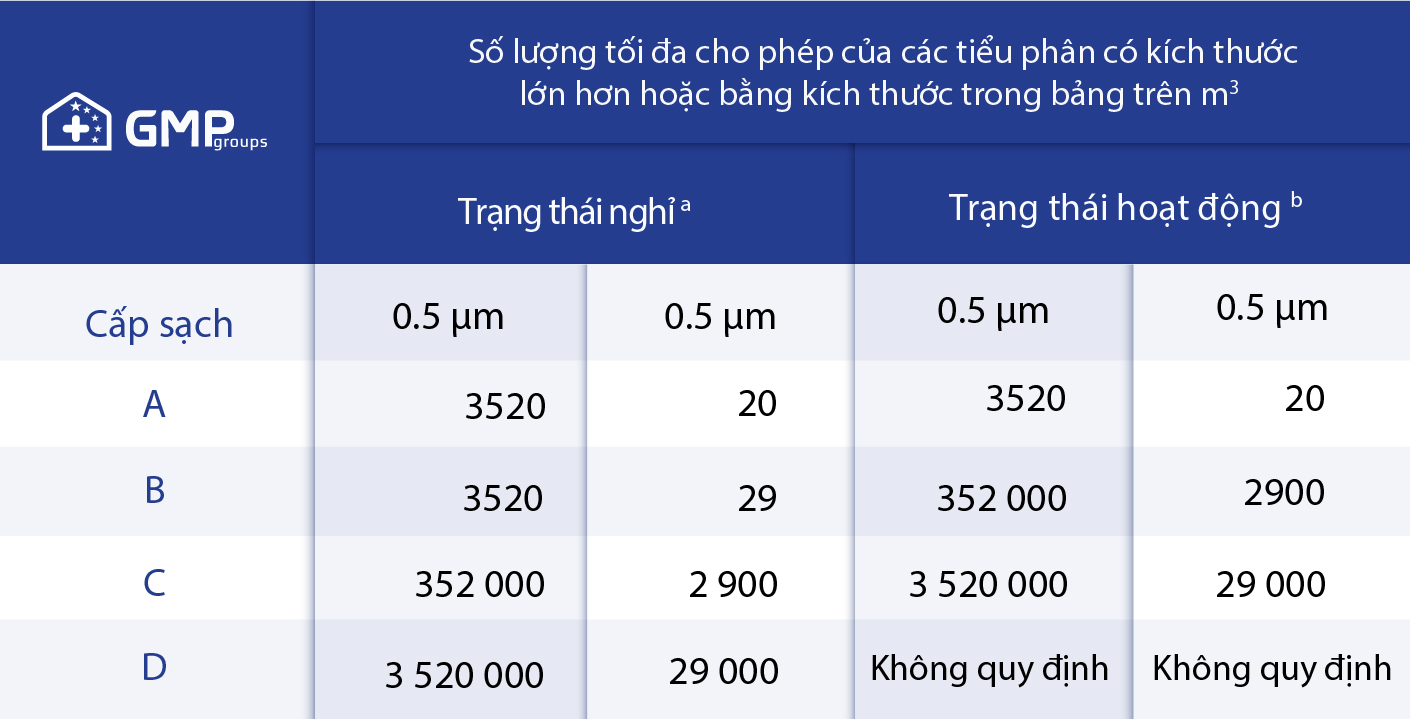
In the production process, the environment is the factor that most affects the quality of veterinary medicines. Depending on the different stages of production, the level of environmental control will vary. In which all stages where the environment is exposed to the product must be strictly controlled. In order to control the environment at the production stages exposed to the product, the environment must be in a safe and quality “clean room” space.
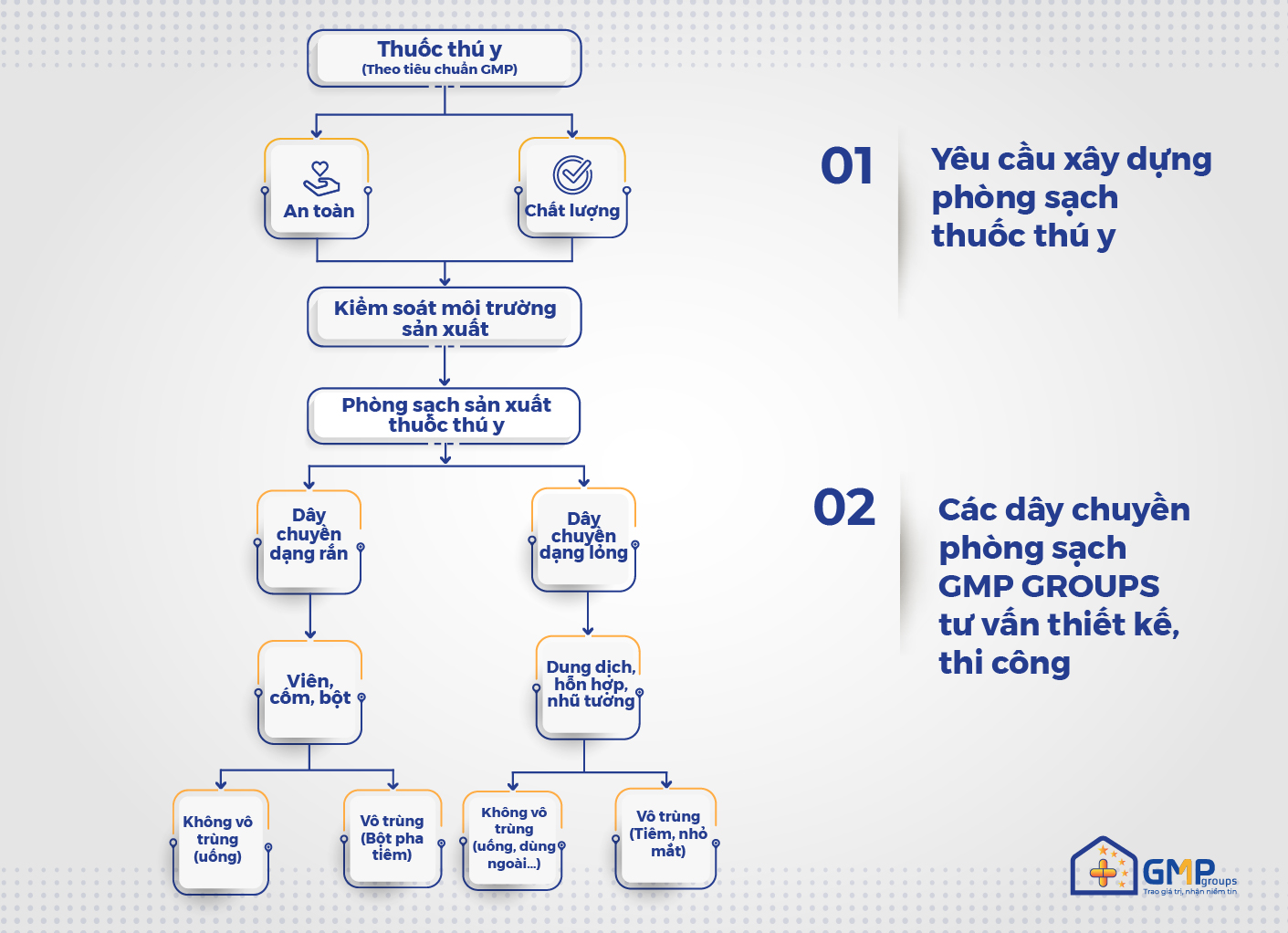
2.2. Cleanroom line for veterinary medicines designed and constructed by GMP Groups
GMP Groups advises on the design and construction of a number of veterinary drug production lines to ensure GMP standards as follows:
– Liquid line: solution, emulsion mixture
+ Non-sterile medicines: oral form, use outside …
+ Sterile drugs: injections, eye drops …
– Solid line: pellets, nui and powder
Non-sterile medicines: oral form
+ Sterile medicine: injectable powder
*Who technical report series, No. 961, 2011 – Good practice of sterile pharmaceutical manufacturing.
Here’s an overview of GMP Groups’ veterinary medicine cleanroom service. For more information, please see more at: What is a veterinary cleanroom? Requirements, standards of veterinary cleanroom
With the desire to bring a comprehensive service ecosystem related to the field of veterinary medicine production, GMP Groups will be the perfect choice for any customer’s needs.
For more information on CONSULTING – DESIGN – CONSTRUCTION OF CLEANROOM, please contact:
GMP Groups Joint Stock Company
Head office: Lot LK20.8, Ecoriver Eco-Investor, Hai Tan Ward, Hai Duong City, Hai Duong Province
Hotline: 0945.255.457
Website: gmpgroups.com.vn
Email: info@gmpgroups.com.vn